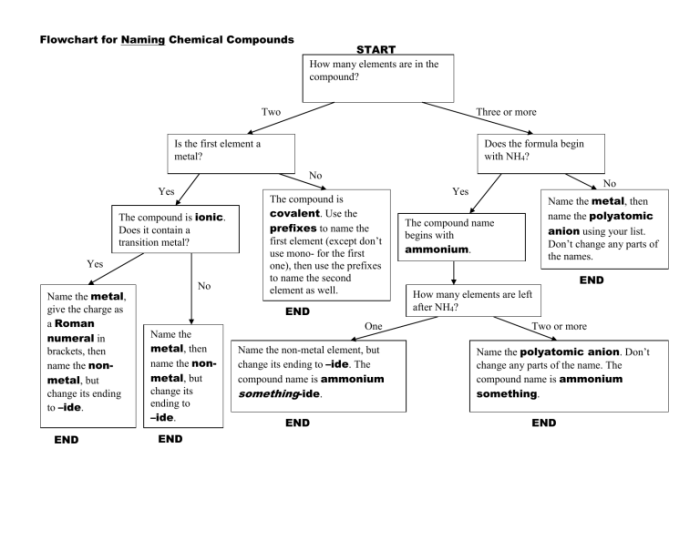
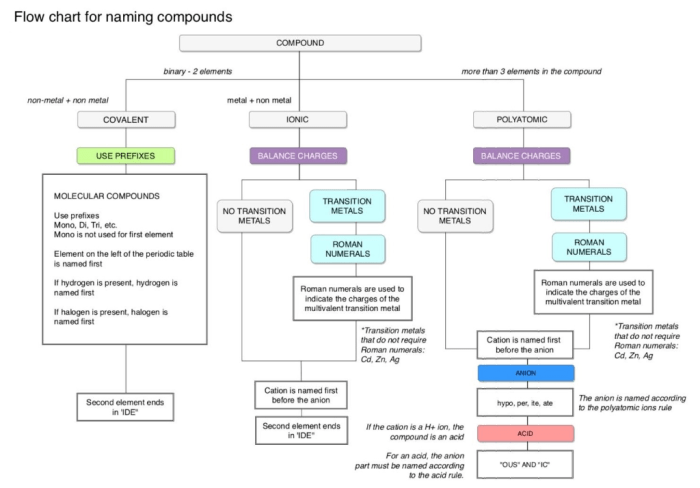
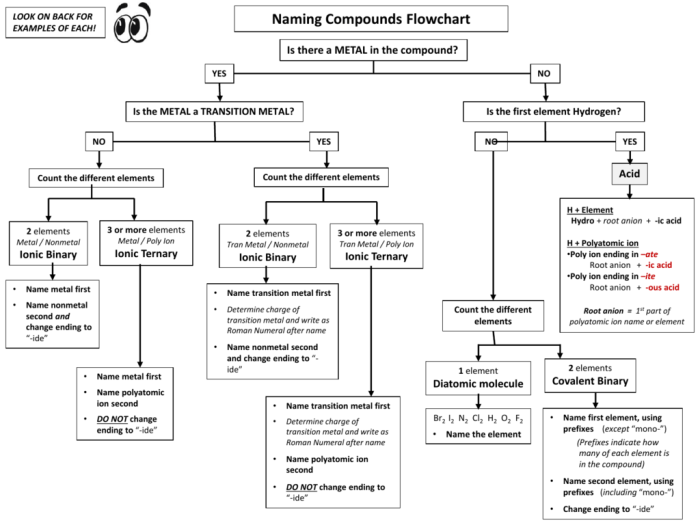
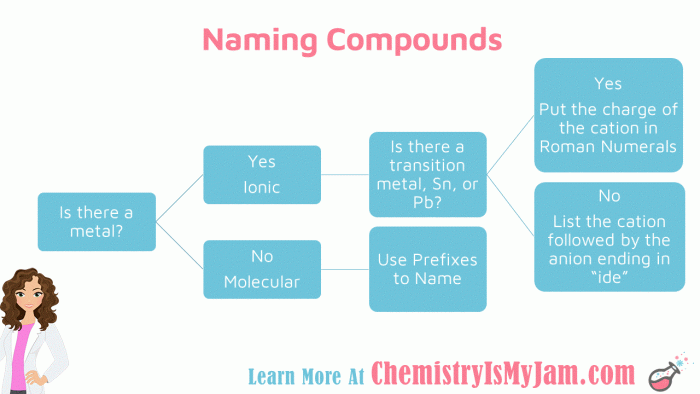
Embark on a comprehensive exploration of the flowchart for naming ionic compounds, a systematic approach that unravels the intricacies of chemical nomenclature. This guide unveils the fundamental principles and practical steps involved in accurately naming these essential building blocks of matter, empowering students and practitioners alike.
Through a lucid and engaging narrative, we delve into the significance of ionic compounds, the rationale behind their systematic naming, and the invaluable role of flowcharts in streamlining this process. Get ready to master the art of ionic compound nomenclature with clarity and confidence!
Introduction
Ionic compounds are formed when a metal loses one or more electrons to a non-metal. The metal becomes a positively charged ion (cation), and the non-metal becomes a negatively charged ion (anion). The oppositely charged ions are attracted to each other by electrostatic forces, forming an ionic bond.The
naming of ionic compounds follows specific conventions. The cation is named first, followed by the anion. The name of the cation is the same as the name of the metal. The name of the anion is derived from the root name of the non-metal, with the suffix “-ide”.Using
a flowchart for naming ionic compounds can be helpful because it provides a step-by-step guide to the naming process. This can help to ensure that the compound is named correctly and consistently.
Step-by-Step Flowchart
To effectively name ionic compounds, a step-by-step flowchart provides a structured approach. This flowchart Artikels the essential steps involved in determining the correct name for a given ionic compound, ensuring accuracy and consistency in naming conventions.
Flowchart
| Step-by-Step Flowchart for Naming Ionic Compounds | |
|---|---|
| Step 1: Identify the Cations and Anions | Determine the individual ions present in the compound, including their charges. |
| Step 2: Write the Cation Name First | The cation (positively charged ion) is named first, followed by the anion (negatively charged ion). |
| Step 3: Name the Cation | Use the element’s name followed by the suffix “-ium” for metals. |
| Step 4: Name the Anion | Use the root of the element’s name followed by the suffix “-ide” for non-metals. |
| Step 5: Add Prefixes for Multiple Ions | If there are multiple ions of the same type, use prefixes (mono-, di-, tri-, etc.) to indicate the number of ions. |
| Step 6: Balance the Charges | Ensure that the total positive charge of the cations equals the total negative charge of the anions. |
| Step 7: Finalize the Compound Name | Combine the cation and anion names to form the complete ionic compound name. |
By following these steps systematically, the correct name for an ionic compound can be determined efficiently and accurately.
Naming Cations and Anions
Ionic compounds are formed when a metal loses one or more electrons to a nonmetal. The resulting positively charged ion is called a cation, while the negatively charged ion is called an anion. The names of cations and anions follow specific rules.
Naming Cations
The rules for naming cations are as follows:
- For cations of metals with only one common oxidation state, the name of the cation is the same as the name of the metal. For example, Na+ is called the sodium ion.
- For cations of metals with multiple common oxidation states, the name of the cation includes the name of the metal followed by a Roman numeral in parentheses indicating the oxidation state. For example, Fe2+ is called the iron(II) ion, and Fe3+ is called the iron(III) ion.
Naming Anions, Flowchart for naming ionic compounds
The rules for naming anions are as follows:
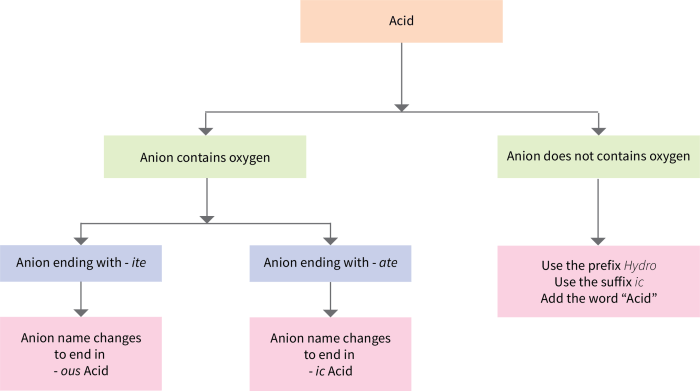
- For anions of nonmetals, the name of the anion is the root of the nonmetal’s name followed by the suffix -ide. For example, Cl- is called the chloride ion.
- For anions of oxyanions, the name of the anion includes the root of the nonmetal’s name followed by the suffix -ite or -ate. The suffix -ite is used for anions with a lower oxidation state, and the suffix -ate is used for anions with a higher oxidation state.
For example, NO2- is called the nitrite ion, and NO3- is called the nitrate ion.
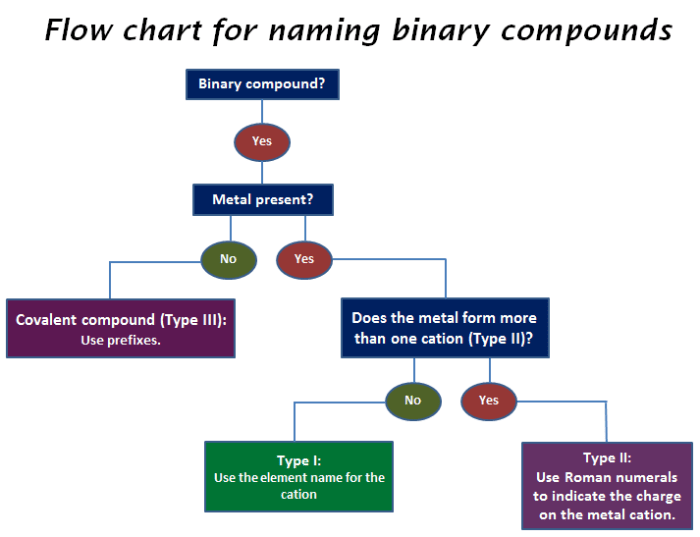
Binary Ionic Compounds
Binary ionic compounds are composed of two elements: a metal and a nonmetal. The metal loses one or more electrons to achieve a stable electron configuration, becoming a positively charged ion called a cation. The nonmetal gains the electrons lost by the metal, becoming a negatively charged ion called an anion.
To name a binary ionic compound, first identify the metal and nonmetal elements present. The name of the metal is written first, followed by the name of the nonmetal with the suffix “-ide”. For example, the binary ionic compound formed between sodium (Na) and chlorine (Cl) is named sodium chloride (NaCl).
Variable Charge Cations
Some metal ions can have multiple charges, known as variable charge cations. When naming binary ionic compounds with variable charge cations, the charge of the cation must be specified using Roman numerals in parentheses after the metal name. For example, iron (Fe) can form two common cations: Fe 2+and Fe 3+. The binary ionic compound formed between iron(II) and oxygen (O) is named iron(II) oxide (FeO), while the compound formed between iron(III) and oxygen is named iron(III) oxide (Fe 2O 3).
Polyatomic Ions: Flowchart For Naming Ionic Compounds
Polyatomic ions are charged species composed of two or more atoms that are covalently bonded. They have a net positive or negative charge and behave as a single unit in chemical reactions.
Polyatomic ions have specific names and charges that must be memorized. The following table lists some common polyatomic ions and their corresponding names and charges:
Common Polyatomic Ions
| Name | Formula | Charge |
|---|---|---|
| Ammonium | NH4+ | +1 |
| Hydroxide | OH– | -1 |
| Nitrate | NO3– | -1 |
| Nitrite | NO2– | -1 |
| Sulfate | SO42- | -2 |
| Carbonate | CO32- | -2 |
| Phosphate | PO43- | -3 |
When naming ionic compounds containing polyatomic ions, the name of the cation is written first, followed by the name of the polyatomic ion. For example, the ionic compound NaCl is named sodium chloride, and the ionic compound CaCO 3is named calcium carbonate.
Naming Complex Ions
Complex ions are ions that contain a metal ion surrounded by a group of ligands. Ligands are molecules or ions that donate a pair of electrons to the metal ion. The name of a complex ion includes the name of the metal ion, the names of the ligands, and the oxidation state of the metal ion.
The oxidation state of a metal ion is the charge that the metal ion would have if all of the ligands were removed. The oxidation state of the metal ion is written as a Roman numeral after the name of the metal ion.
Naming Ligands
Ligands are named according to the number of atoms they donate to the metal ion. The most common types of ligands are:
- Monodentate ligands: These ligands donate one atom to the metal ion.
- Bidentate ligands: These ligands donate two atoms to the metal ion.
- Tridentate ligands: These ligands donate three atoms to the metal ion.
- Tetradentate ligands: These ligands donate four atoms to the metal ion.
The names of ligands are often derived from the Greek words for the number of atoms they donate. For example, “mono” means one, “di” means two, “tri” means three, and “tetra” means four.
Examples of Naming Complex Ions
- [Fe(H 2O) 6] 3+: Hexaammineiron(III) ion
- [Co(NH 3) 4Cl 2] +: Tetraamminedichlorocobalt(II) ion
- [Pt(en) 2Cl 2] 2+: Dichlorodiethylenediamineplatinum(II) ion
Essential Questionnaire
What is the purpose of a flowchart for naming ionic compounds?
A flowchart for naming ionic compounds provides a step-by-step visual guide that simplifies the process of assigning systematic names to these compounds, ensuring accuracy and consistency.
How does the flowchart account for variable charge cations?
The flowchart incorporates specific steps for identifying and naming cations with variable charges, utilizing Roman numerals to indicate the charge state.
What is the significance of polyatomic ions in the flowchart?
The flowchart includes a comprehensive table of common polyatomic ions, along with their names and charges, enabling the accurate naming of ionic compounds containing these complex ions.