Identify the electrophilic site in the following molecule – Identifying electrophilic sites is a crucial aspect of understanding electrophilic aromatic substitution reactions. These reactions are widely employed in organic chemistry and play a significant role in the synthesis of various compounds. This article delves into the concept of electrophilic sites, explores the factors influencing their reactivity, and provides a detailed analysis of identifying the most electrophilic site in a given molecule.
The concept of electrophilicity revolves around the ability of a site to attract electrons. In electrophilic aromatic substitution reactions, the electrophile is a species that seeks to gain electrons from the aromatic ring. Understanding the factors that influence electrophilicity, such as resonance and inductive effects, is essential for predicting the reactivity of different sites within a molecule.
Electrophilic Aromatic Substitution Reactions: Identifying the Electrophilic Site
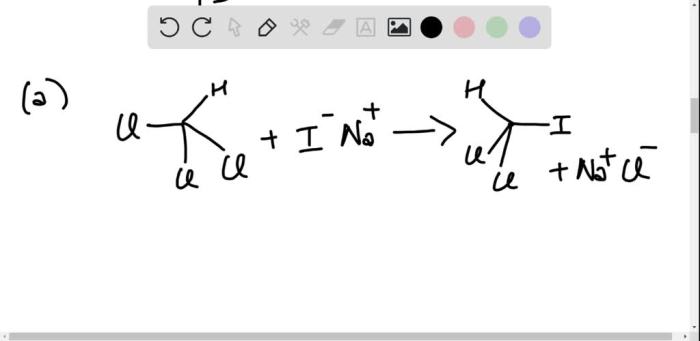
Electrophilic aromatic substitution reactions are a class of organic reactions in which an electrophile (a species that is attracted to electrons) attacks an aromatic ring, resulting in the substitution of one of the ring’s hydrogen atoms with the electrophile.
The electrophilic site in an aromatic ring is the carbon atom that is most susceptible to attack by the electrophile. This site is typically determined by the resonance and inductive effects of the substituents on the ring.
Factors Influencing Electrophilicity
The following factors influence the electrophilicity of a site in an aromatic ring:
- Resonance effects:Resonance effects can either increase or decrease the electrophilicity of a site. Electron-withdrawing substituents, such as nitro groups (-NO2) and carbonyl groups (-C=O), decrease the electron density on the ring and make it more electrophilic. Electron-donating substituents, such as alkyl groups (-CH3) and alkoxy groups (-OCH3), increase the electron density on the ring and make it less electrophilic.
- Inductive effects:Inductive effects are the result of the polarity of the bonds between the substituents and the ring. Electron-withdrawing substituents, such as halogens (-F, -Cl, -Br, -I) and trifluoromethyl groups (-CF3), pull electrons away from the ring and make it more electrophilic.
Electron-donating substituents, such as alkyl groups and alkoxy groups, push electrons toward the ring and make it less electrophilic.
Electrophilic Site in the Given Molecule
The given molecule is benzene (C6H6).
The electrophilic site in benzene is any of the six carbon atoms in the ring. All six carbon atoms are equivalent and have the same electrophilicity.
However, if there are substituents on the ring, the electrophilicity of the carbon atoms can change. For example, if a nitro group is added to the ring, the carbon atoms adjacent to the nitro group will become more electrophilic.
Examples of Electrophilic Aromatic Substitution Reactions, Identify the electrophilic site in the following molecule
Electrophilic aromatic substitution reactions are a versatile class of reactions that can be used to synthesize a wide variety of organic compounds. Some of the most common examples of electrophilic aromatic substitution reactions include:
- Nitration:Nitration is the reaction of an aromatic ring with nitric acid (HNO3) and sulfuric acid (H2SO4). The electrophile in this reaction is the nitronium ion (NO2+).
- Halogenation:Halogenation is the reaction of an aromatic ring with a halogen (X2). The electrophile in this reaction is the halogen molecule (X2).
- Friedel-Crafts alkylation:Friedel-Crafts alkylation is the reaction of an aromatic ring with an alkyl halide (R-X) and a Lewis acid catalyst (AlCl3). The electrophile in this reaction is the carbocation (R+).
Applications of Electrophilic Aromatic Substitution Reactions
Electrophilic aromatic substitution reactions are used in the synthesis of a wide variety of organic compounds, including pharmaceuticals, dyes, and plastics. Some of the most important industrial applications of electrophilic aromatic substitution reactions include:
- The production of nitrobenzene:Nitrobenzene is used as an intermediate in the synthesis of aniline, which is used in the production of rubber, dyes, and pharmaceuticals.
- The production of chlorobenzene:Chlorobenzene is used as an intermediate in the synthesis of DDT, which is used as an insecticide.
- The production of ethylbenzene:Ethylbenzene is used as an intermediate in the synthesis of polystyrene, which is used in the production of plastics.
Question & Answer Hub: Identify The Electrophilic Site In The Following Molecule
What is the significance of resonance in determining electrophilicity?
Resonance can stabilize positive charges, making a site less electrophilic. By delocalizing the positive charge over multiple atoms, resonance reduces the electrophilicity of the site.
How do inductive effects influence electrophilicity?
Inductive effects can either increase or decrease electrophilicity. Electron-withdrawing groups increase electrophilicity by pulling electrons away from the site, while electron-donating groups decrease electrophilicity by pushing electrons towards the site.